- Home
- Prenatal diagnosis
- Prenatal Diagnosis Techniques
Prenatal Diagnosis Techniques
If we are to perform this series of tests during the course of the pregnancy, we will logically have to employ techniques that will allow us to gain access into the foetus. At present, these techniques are divided into invasive and non-invasive techniques of the foetal space.
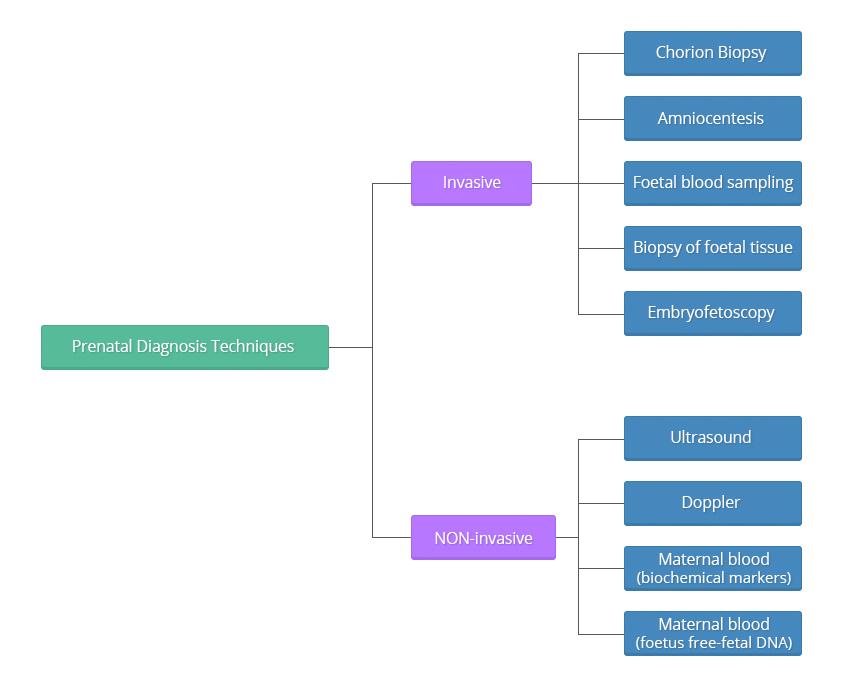
Invasive techniques
Invasive techniques are defined as those that invade or physically enter the foetal space or surroundings, for example, puncturing the amniotic sack to withdraw fluid. These tests must be performed observing very strict sterility measures.
The difference among these techniques is the time or weeks of gestation at which they are performed during the pregnancy; the reliability of the tests, as they all present an error margin (remember that in medicine there is never 0 or 100, we always talk in terms of probability); and the risk of miscarriage, which varies depending on each particular technique.
These techniques should be performed at specific times throughout the gestational period in order to increase the possibility of getting a reliable result while, at the same time, decreasing the risk of miscarriage, and should always be performed under ultrasound guidance.
The adoption of a specific technique will depend on the type of diagnosis required.
In all cases, and whenever possible, the safest technique with the fastest results will be used. For obvious reasons, it is different to inform the patient of a pathological finding at 11 weeks than it is when she is already into her 22 week.
If chromosomal determinations are required, any of these techniques will be appropriate. However, chorionic biopsy and amniocentesis are the most recommendable tests, as they can both be performed early in the pregnancy and provide the greatest experience.
- Chorion Biopsy
Chorion Biopsy
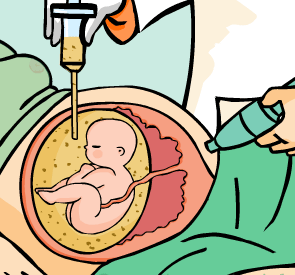
A chorion biopsy is the collection of villi from the chorionic area of the growing placenta. This type of biopsy should always be performed under ultrasound guidance, and may either performed transcervically or transabdominally using a catheter or a clamp appropriate to the gestational age.
The test must be performed after the 11th week of pregnancy to term. If the test if performed before the 10th week, it can lead to abnormalities of the fetal limbs, micrognathia and microglossia. For purposes of prenatal diagnosis of chromosomal defects or inherited diseases for DNA alterations, the optimum gestational age to carry out the procedure is between the 11th and 12th weeks of pregnancy.
Chorionic villi provide excellent material to conduct DNA molecular studies and enzymatic determinations.
The risk of miscarriage due to the technique is unknown, as in this gestational period, many spontaneous abortions occur. It remains unknown whether any of the women who underwent the test would have also ended in miscarriage, should they not had the test. Presently, this risk is estimated about 1%.
Transcervical approach
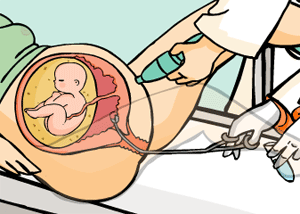
The catheter is inserted through the vagina and uterine neck into the uterus to the edge of the placenta where the chorionic villi is snipped or suctioned off for diagnosis.
Transabdominal approach
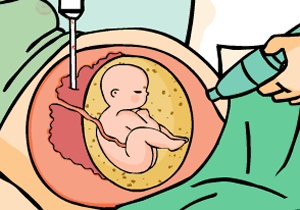
In the transabdominal procedure, a needle is inserted through the abdomen to the edge of the placenta from where the chorionic villi will be obtained.
- Amniocentesis
Amniocentesis
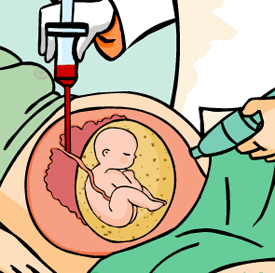
This procedure consists in the extraction of amniotic fluid through a needle that has been inserted transabdominally under ultrasound guidance. For purposes of chromosomal or genetic disorders diagnosis, amniocentesis is habitually performed between the 14th and 20th weeks of pregnancy, (ideally between 15 and 17) obtaining about 15 to 20 ml of amniotic liquid. It has been shown that if the test is performed earlier in the pregnancy, the uterus is not as accessible and the number of foetal cells is scarce, and if performed later in the pregnancy, we run the risk that many of the cells obtained are keratinised and unfeasible for study.
If the test is performed between the 15th and 20th week of pregnancy the risk of spontaneous abortion is less than 1%.
Amniocentesis is also possible at 10-14 weeks of gestation. If the test is performed during these earlier weeks, the risk of spontaneous abortion increases around 2%, and there is a higher incidence of talipes equinovarus (foot abnormality), as well as rupture of the membranes; hence, although technically possible, this procedure is NOT recommended and should only be used in very specific cases and with the patient´s consent.
Complications of amniocentesis:
Spontaneous abortions (miscarriages) = < 1%
Contamination of the amniotic fluid by stem cells = 0.15 – 0.11%.
Loss of amniotic fluid = 1 – 2 %.
Blood loss, with scanty “spotting”, very rare.Complications with amniocentesis are rare under adequate asepsis. However, one of the unlikely complications would be an infection in the mother.
- Foetal blood sampling
Foetal blood sampling
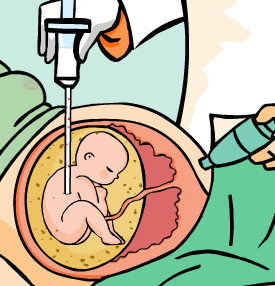
This test obtains foetal blood by direct puncture of the funicular vessels under ultrasound or foetoscopic guidance.
The main indications of this technique include: Rapid assessment of foetal chromosomes (results can be obtained within 24 to 48 hours), immunological assessment of the foetus, and assessment of certain haemopathies or blood disorders.This test is usually performed after the 20th week of pregnancy through delivery. It is not advisable to perform this test in the early stages of pregnancy because blood vessels in the umbilical cord are very small at this stage of development and can get damaged when they are punctured to extract the blood.
Normally between 0.5 and 4 ml of foetal blood are collected. The sample is analysed immediately to make sure it is foetal blood, as the maternal placental blood vessels are in close proximity to where the sample is collected and an error could easily occur.
The risk of spontaneous abortion as a result of the test is approximately 1-3%.
- Biopsy of foetal tissue
Biopsy of foetal tissue
This technique is used to obtain foetal tissues for specific testing or screening, such as skin, liver or muscular biopsies.
As in the previously described techniques, the procedure is always carried out under ultrasound or foetoscopic guidance. - Embryofetoscopy
Embryofetoscopy
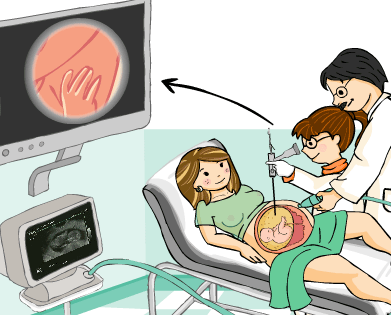
Embryofetoscopy consists in the direct visualisation of the embryo or fetus during pregnancy through a device called endoscope or fetoscope.
- When this technique is performed during the first 12 weeks of pregnancy, it is called embryoscopy.
- When performed after the 12th week of pregnancy, it is called fetoscopy.
Embryofetoscopy is used for therapeutic and diagnostic purposes only when it is NOT possible to obtain the same results using ultrasound or ultrasound combined with other prenatal diagnosis techniques such as chorion biopsy, amniocentesis or funiculocentesis, due to the risks involved for both the mother and her unborn child. These risks can include: a 12% risk of miscarriage, bleeding, infection, liquid loss, sensitivisation problems caused by paternal Rh- incompatibility if adequate prevention measures are not taken, and a 47% risk of premature rupture of the membranes.
Consequently, embryofetoscopy should only be carried out in specialised centres with qualified professionals trained in this sort of procedures and with the appropriate equipment (fetoscopes, with specific instruments designed for each task, video cameras, digital cameras, light sources and energy, etc.).
Embryofetoscopy should always be performed under ultrasound guidance.
Diagnostic uses
Embryofetoscopy detects congenital malformations and hereditary diseases which can only be diagnosed by:
- Direct external visualisation, for instance in genodermatosis (hereditary diseases of the skin where molecular biology studies cannot be carried out).
- Morphological visualisation of some external anomalies suspected during the ultrasound examination in the early phases of pregnancy.
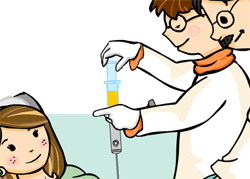
Collection of foetal blood samples or amniotic fluid samples.
Administration of drugs or therapeutic agents to the foetus.
Therapeutic uses
Fetoscopy enables us to carry out explorations of the foetus, umbilical cord, placenta or membranes.
Most indications on the foetus are still the subject of research with the hope that the applications will help us to save their lives or prevent irreversible sequelae: shunt placement, in cases of obstructive processes, destructions of amniotic bands, treatment for the premature rupture of membranes, tracheal occlusions for the treatment of diaphragmatic congenital hernias, treatment of sacrococcigeous teratomas, treatment of placental chorioangiomas, cord occlusions to carry out selective feticides in monochorial twins, fulguration of posterior urethral valves in lower urinary obstruction, repair of myelomeningocele, etc…
At present, the main application of this technology for therapeutic aims is applied in the treatment of twin-to-twin transfusion syndrome.
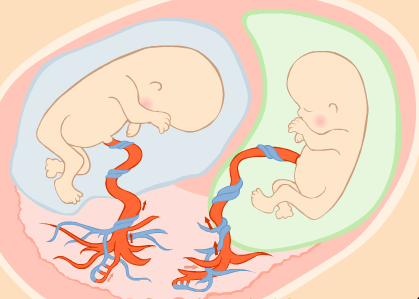
This syndrome is a disease of the placenta that affects mainly about 15% of monochorial twins (foetuses that have evolved from one single ovum and spermatozoon and that share the same placenta) due to the formation of abnormal vascular placental connections between the two twins.
Under normal conditions, each twin has its own balanced circulatory system.
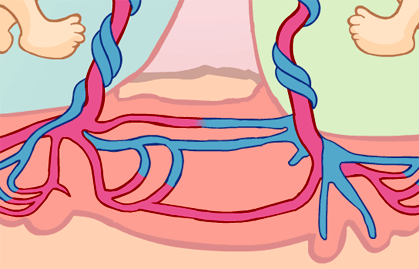
However in this kind of twins is frequently that they establish connections between them. There can be different types of vascular connections: between arteries, between veins or artery-to-vein.
Amongst these connections, artery-to-vein connections can be the most problematic, depending on whether they are balanced or unbalanced.
Balanced connections
Unbalanced connections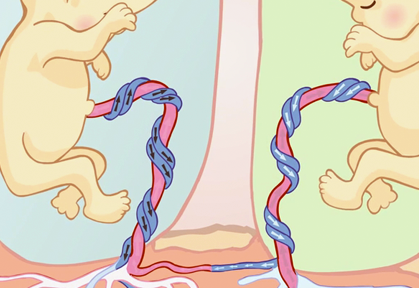
When these connections are balanced, there is no problem (as the blood flow is also in balance and both foetuses are donors and recipient at the same time).
A twin can die prenatally because the recipient foetus has an overloaded circulatory system which can lead, among other complications (polyhydramnios) to heart failure, whereas the donor foetus develops a severe anaemia that prevents growth.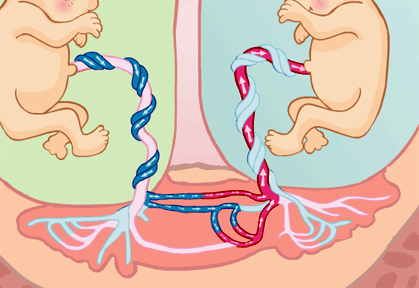
On occasions, if the blood flows in one single direction because it is the blood of one of the twins passing to the other through pathological connections, there is a haemodynamic unbalanced known as twin-to-twin transfusion syndrome. In this instance, one of the foetuses is the donor and the other the recipient.
If the condition is not diagnosed and corrected in time, both foetuses run the risk of dying, either before (prenatally) or after birth (postnatally).
A twin can die postnatally because of premature birth or “prematurity”. Some of those who survive (10%) can develop very serious neurological problems among them brain paralysis.
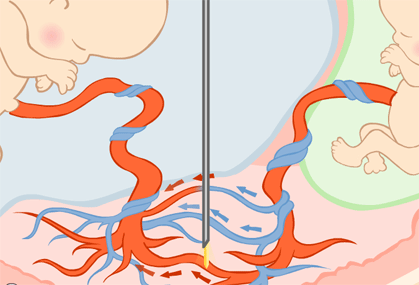
At the present time, the best treatment consists in the identification and coagulation of the abnormal vascular connections by means of laser endoscopic surgery that blocks the passage of blood from one twin to the other.
NON-Invasive techniques
As regards non-invasive techniques, these procedures do not pose any risk of abortion. Non-invasive diagnostic methods include: Methods that employ ultrasonic waves such as the ULTRASOUND and the DOPPLER examination, which can be safely used at any time during the pregnancy through delivery. And blood chemistry tests using MATERNAL BLOOD (maternal blood testing) during the first and second trimesters of the pregnancy.
- Ultrasound
Ultrasound
An ULTRASOUND or echography uses ultrasonography or “sound waves” as a diagnostic tool. An ultrasound sends waves to the foetus that bounce off internal foetal structures (thereby its name “echo”). It allows visualisation of the foetus in a TV-like viewing screen, providing internal as well as external images of the foetus. Internal and external malformations can be diagnosed based on different tissue densities. It works in the same way as radars in air traffic control or marine navigation.
Given the constant progress being made in this field, it is now possible to explore the foetus in a bi-dimensional plane (2D) or in a three-dimensional plane, either in a static (3D) or in movement (4D). The use of this technique requires:- Highly qualified professionals in this field.
- The use of devices equipped with the latest technology and approved by the competent regulatory authorities.
View 2D ultrasound on Youtube View 3D ultrasound on Youtube View 4D ultrasound on Youtube
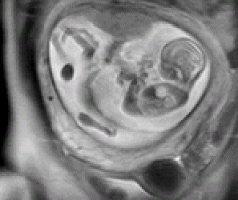 Occasionally, it is necessary to employ other diagnostic techniques to complement the ultrasound diagnosis, such as magnetic nuclear resonance. This technique employs state of the art devices for rapid image acquisition and storage (which is ideal for the foetus as it is in constant motion within the womb) and allows us to perform an anatomical foetal tracing, which is very useful to confirm certain pathological conditions of the central nervous system, as well as other thoracic and renal pathologies. This image shows a foetus with a diaphragmatic hernia; this means that its lung capacity is partly occupied by intestinal content.
Occasionally, it is necessary to employ other diagnostic techniques to complement the ultrasound diagnosis, such as magnetic nuclear resonance. This technique employs state of the art devices for rapid image acquisition and storage (which is ideal for the foetus as it is in constant motion within the womb) and allows us to perform an anatomical foetal tracing, which is very useful to confirm certain pathological conditions of the central nervous system, as well as other thoracic and renal pathologies. This image shows a foetus with a diaphragmatic hernia; this means that its lung capacity is partly occupied by intestinal content.
The series of images shown next correspond to a girl’s face with a mouth tumour (epulis) studied using the previously mentioned techniques.
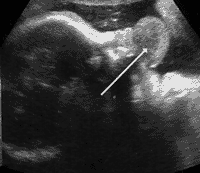
Conventional ultrasound 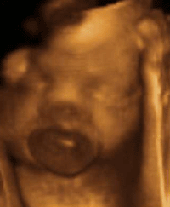
Three-dimensional ultrasound 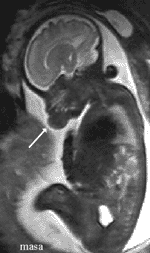
Magnetic resonance You can see some images of the newborn girl before and after the tumor removal (epulis). Viewer discretion is advised. View surgery pictures 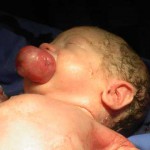
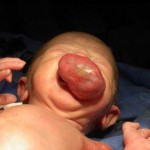
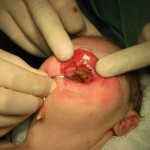

Girl picture at one month oldThis association of techniques for certain malformations permits us to suspect the aetiology of these malformations, to determine the type of tissue or tissues involved; and to establish the prognosis of the alteration, should it be left to run its natural course, and based on this, to determine:
- What is the best time to induce labor, as some malformations can compromise the life of the child, or if allowed to overgrow they may produce irreversible lesions in the actual organ or surrounding tissues.
- To plan the birth, that is a vaginal delivery or by means of a C-section.
- To have a perinatology and/or surgical team ready at the Labour and Delivery Room for the immediate care of the newborn.
- Doppler
Doppler
A DOPPLER also uses sound waves as a diagnostic tool, but the waves are sent to the foetal blood instead, allowing us to study the placental flow and the foetal circulatory system. By assessing the path followed by the blood in the organs, malformations or alterations of the circulatory system can be easily diagnosed.
- Maternal blood (biochemical markers)
Maternal blood tests
 In these tests, a sample of the maternal blood is taken during pregnancy to quantify a series of substances segregated by the foetus or placenta. This assessment allows us to identify pregnant women who are at risk of having a baby affected with some type of chromosomal condition (trisomy 13, 18, 21 (this latter one is also known as Down’s syndrome or mongolism), Turner’s syndrome (45, X), triploidy, etc. Or certain malformations, among these defects of the neural tube such as spina bifida (also known as open dorsal spine) and anencephaly.
In these tests, a sample of the maternal blood is taken during pregnancy to quantify a series of substances segregated by the foetus or placenta. This assessment allows us to identify pregnant women who are at risk of having a baby affected with some type of chromosomal condition (trisomy 13, 18, 21 (this latter one is also known as Down’s syndrome or mongolism), Turner’s syndrome (45, X), triploidy, etc. Or certain malformations, among these defects of the neural tube such as spina bifida (also known as open dorsal spine) and anencephaly.
These test should be performed at specific weeks of pregnancy because their values change as the pregnancy progresses and if these tests are performed outside these times, they may no longer be of informative value. At present, these tests are performed during the first and second trimesters, and, among all the available markers, the most commonly tested ones are:
- During the first trimester (8 to 13 weeks of pregnancy, ideally during the 10th week).
- B-hCG (free beta fraction of human chorionic gonadotropin) and PAPP-A (pregnancy associated plasma protein A) levels are determined.
- During the second trimester (14 to 20 weeks of pregnancy, ideally during the 16th week): this test can be of three different types depending on whether two, three or four substances are measured; in the first case we will be talking about double screening, in the second case, a triple screening and in the third case, a quadruple screening.
- Double screening: This test measures the concentrations of two substances: alpha-fetoprotein AFP and hCG (total or free-beta).
- Triple screening: This test measures the concentrations of three substances, namely AFP, hCG (total or free-beta), non-conjugated estriol uE3.
- Quadruple test: This test measures the concentrations of four substances AFP, hCG (total or free beta), uE3 and inhibin-A.
Before reporting the results, the laboratory should assess the results obtained taking into account the mother’s age and a series of factors that have the capacity to modify the levels of these substances such as the mother’s weight, the race, smoking, insulin-dependent diabetes, the number of foetuses carried in the pregnancy (twins, triples, etc.)
At present, these biochemical tests are complemented with ultrasound studies to increase their detection rate. Subsequently, and taking into account these two results (biochemical + ultrasound examinations), together with the clinical history and the mother’s age, the individual risk is calculated for each individual patient. It must be made clear that if the test yields an abnormal result, it does NOT mean that a chromosomal alteration has been diagnosed, given that these tests are not of diagnostic value.
These tests only alert us about the POSSIBILITY of existence of some type of alteration. Consequently, once the results become available, the physician will determine whether further tests are necessary (chorion biopsy, amniocentesis, etc.).Now, let’s focus on the ECHOGRAPHIC MARKERS. Echographic markers are assessed when fetal echographic examinations are carried out during the first and second trimester of pregnancy.
First trimester
There are several ultrasound markers used to detect chromosomal disorders during the FIRST TRIMESTER as each abnormality follows its own syndromic pattern of detectable alterations (megalocystis, single umbilical artery, crown-rump length, etc.). Nevertheless, given its great importance in the detection of the most frequently occurring chromosomal alterations (trisomy 13, 18, 21 and Turner’s syndrome (45,X), we will rule out the following.
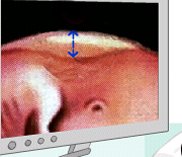
Nuchal translucency (NT)
An ultrasound marker consisting in the measurement of the physiological accumulation of liquid in the foetal neck while pregnancy is between 8 to 14 weeks.
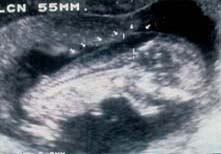
This is a pathological translucency. The risk of a chromosomal defect starts after a given thickness.
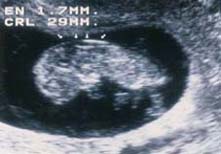
This is a normal translucence
Assessment of presence/ absence of foetal nasal bone
The nasal bone is commonly visualised during an ultrsound examination. Nonetheless, given that size of the nose is an thnic feature, it may be not possible to visualise this structure in normal foetuses. The incidence of this occurrence is 1% for Caucasians, 10% for African-Caribbean, and 7% for Asiatics.
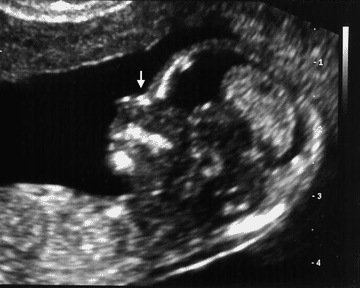 Presence of the nasal bone (please observe the two whitish structures making a = sign and the tip of the nose at the most prominent end)
Presence of the nasal bone (please observe the two whitish structures making a = sign and the tip of the nose at the most prominent end)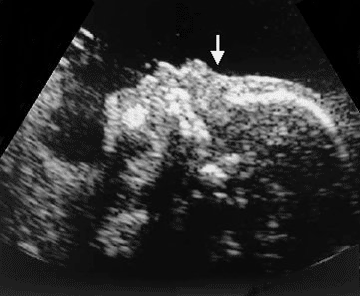
Absence of the nasal bone (a whitish structure which is the tip of nose is only observed)
On the other hand, the nasal bone is absent in 60-70% of foetuses with trisomy 21, in approximately 50% of the foetuses with trisomy 18 and in 30% of foetuses with trisomy 13.
A Doppler study of blood vessels and the foetal heart rate
The ductus venosus study (blood vessel that transports most of the venous blood from the placenta to the heart) is especially sensitive, given that when it is affected, we should suspect the presence of certain chromosomal alterations as well as cardiac malformations.
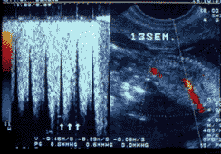
Normal ductus venosus
Pathological ductus venosus: Please notice that the images transmitted by the blood flow wave are different to those of a normal pattern
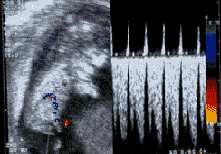
Doppler assessment of tricuspid insufficiency
Tricuspid insufficiency occurs in 65% of foetuses with trisomy 21 and in 6% of foetuses who have healthy chromosomes.
Assessment of the craniomaxillofacial angle
An upper facial angle of 85º is present in 65% of cases of foetuses with trisomy 21 and in 5% of chromosomically normal foetuses. The most commonly used echographic marker in the first trimester nuchal translucency, whereas the rest of markers are usually used when nuchal translucency results reach borderline values or pregnancies at very high risk of chromosome pathology. Just so that you get an idea, the screening for trisomy 21 in the first trimester (which combines ultrasound for nuchal translucency and nasal bone + the determination of free Beta-hCG and PAPP-A in maternal blood) allows the clinician to potentially identify over 90% of pregnancies with this syndrom, with a false positive rate of 2,5%.
Second trimester
Several echographic markers are used to detect cromosopathies during the SECOND TRIMESTER and their reliability to calculate the risk of chromosomal alterations is different from each marker.
The assessment of echographic markers i s perfomed between weeks 18 and 22. The most commonly performed test are: the asessment of nuchal fold (measurement of the foetus’s neck thickness which should not generally exceed 6 mm.) and the assessment of cysts in the choroid plexuses being the most commonly performed. Apart from the different types of fetal malformations that might be detected, these malformations, when assessed as a whole, make us suspect specific chromosomal pathologies in certain cases. When a malformation is found during the ultrasound examination, the ultrasound specialist will take a closer, more thorough look into the foetal anatomy in an attempt to find any other accompanying malformations.
It should be made clear that this type of ultrasound examinations should only be carried out by qualified and trained professionals and that the determination of maternal serum markers should only be carried out by duly accredited laboratories.
And please, regardeless of all the information you get, keep in mind that all the information is merely informative and should be correctly interpreted. It is very important that you do not get alarmed and that before taking any decision, you seek the advice of your gynaecologist. If your doctor deems it necessary, he will refer you to a Genetics department, where your particular case will be assessed and all your questions and doubts will be answered, together with a set of instructions to follow in your particular case.
- During the first trimester (8 to 13 weeks of pregnancy, ideally during the 10th week).
- Maternal blood (free-fetal DNA)
HARMONY test (test developed by Ariosa Diagnostics Inc)
The test consists in the analysis of certain genomic sequences of free-fetal DNA found freely circulating in the mother’s bloodstream allowing the diagnosis of the most frequent trisomies: 13 (Patau syndrome), 18 (Edwards syndrome), 21 (Down syndrome ), with a reliability of 92%, 97% and 99% respectively, with a false positive rate of 0.2%, 02%, 0,1%.
All these trisomies are characterized by presenting malformations and mental retardation.
This test is the result of the great advances reached after the sequencing of the human genome in the field of molecular biology. Its potential at present and in the future is impressive.
If the Harmony test shows:
- That there is a low risk for any of these threee trisomies, it is highly likely that the fetus DOES NOT have any of them.
- That there is some risk, then the most likely that the fetus DOES have them. But let’s not forget that these are probabilistic tests, not diagnostic tests.
To perform the test at week 10 of gestation, 20 ml of maternal blood are extracted by means of a venous puncture and it is not necessary to be on an empty stomach.
It is not recommended to perform this test before 10 weeks of gestation because the amount of free-fetal DNA to perform the study is insufficient and it would not be possible to obtain results. In order to be able to perform the test, there must be a minimum of 4% of free-fetal DNA in the mother´s blood.
As research advances in this area, new diagnostic possibilities arise, offering the possibility to perform more advanced genomic testing using the same amount of blood, hence, at present, the following tests are available:
- Study of chromosomes: 13, 18, 21, X, Y.
- Study of chromosomes: 13, 18, 21, X, Y para (aneusomies, trisomies, triploidies), + the most frequent microdeletions: 22q11.2 (Di George syndrome), 1p36, Angelman syndrome, Prader Willy syndrome and Cri du chat syndrome (test also known as extended panorama).
All these pathologies, besides malformations, entail mental retardation.
- Laboratorio de Análisis Echevarne
- Panorama™ Non-Invasive Prenatal Screening for Microdeletion Syndromes
It is recommended that ultrasound examinations be performed at the same time as the screening at weeks 10 and 13 of gestation to rule out malformation or transitory signs associated with chromosome disease and/or assess fetal parameters when interpreting the results.
In 5% of cases it is not possible to get a result, because the sample is not technically correct. This fact has nothing to do with whether the child is OK or not OK, it’s just a technical problem.
The free-fetal DNA analyzed comes from placental cells, and therefore diagnostic errors may exist caused by placental mosaicisms.
It takes from 7 to 14 days to get the results from this test.
All these tests can only be performed in accredited centers accredited for that purpose. The “Fetal Medicine Center” is worldwide known reference center.
You should look for reference centres in your local country where these tests are performed and, this is not possible, you should get the information directly from Ariosa Diagnostics, the laboratory that developed this technique so that they provide you with this information, should they have it.
- The Fetal Medicine Centre
- Fundació Medicina Fetal Barcelona
- Salud de la mujer Dexeus – Maternal Blood test to detect Down syndrome

If you click on the different buttons appearing in the previous illustration, you will find information, animations and links relating to the subject matters. This information will help you understand what each of the techniques is and what it is used for. Most likely, you are asking yourself the following questions:
Related questions
- What kind of tests can I undergo to make sure my baby will be normal?
No one can assure normality 100 per cent.
Each pregnancy is like a lottery, and any disorder of hereditary origin, starts at some point in a given family, despite the fact that no previous family history may exist.
In any case, and as a preventive measure, we recommend:
- BEFORE becoming pregnant, it is highly advisable to go to the gynaecologist to make sure that everything is OK and to rule out any infectious process and assess if you are at any special risk that might be taken into account.
- DURING the pregnancy it is of outmost importance to have regular ultrasound examinations to rule out the presence of malformations and, based on the expectant mother’s age or reproduction history, to conduct any appropriate tests to also exclude any chromosomal or other type of alterations.
- What is genetic counselling?
Genetic counselling is an information process whereby a geneticist, a specialist in human genetics, informs an individual, couple or group of individuals, of the diagnosis, prognosis, treatment, risk and ways to prevent a given disease.
This consultation should only be performed by professionals who have great sensitivity, a sense of humanity, and a good ability to communicate. An easy to understand, clear and concise type of language should always be used, while adopting at all times a non-directive approach.Upon completion of the informative session, the patients or consulting party, after having understood all the information, should be capable of freely making up their own minds based on their wishes and convictions. If this aim is not achieved, then the professional in charge of the counselling session will have failed in his or her mission.
- What is the difference between Prenatal Diagnosis Techniques and Prenatal Detection Techniques?
Quite easy! As their name indicate prenatal diagnostic techniques allow us to identify fetal anomalies of a diverse nature: chromosomal aberrations, biochemical alterations, malformations, etc. whereas prenatal detection techniques allow us to suspect a possible foetal anomaly; in other words:
A detection or screening technique for a given condition is a test that, based on the results, enables us to easily classify patients who have undergone the test into at least two groups: a group at high risk of developing the condition and a group at low risk.
In contrast with diagnostic methods, detection techniques do not draw a clear differentiation between both groups (affected vs unaffected individuals) but rather suggest that in the high risk group there will be some unaffected individuals (false positives) and in the low risk group there will be some affected individuals (false negatives). The more affected individuals the test detects and the fewer cases of false positives it originates, the more effective the test is.
The result of a detection or screening test will never be YES (affected) or NO (unaffected), but will rather assess the probability (likelihood) or risk of being or becoming affected by the disease under study.
Therefore, detection or screening test can never be considered a diagnostic test.
- What Prenatal Diagnosis Techniques are there?
Prenatal diagnosis techniques are those techniques that employ:
- Invasive techniques
- Ultrasound
- What Prenatal Detection or Prenatal Screening Techniques are there?
Detection or screening techniques are techniques that employ:
- Biochemical markers
- Echographic markers
- What types of markers are used to perform detection or screening techniques?
Detection or screening techniques are based on the determination of markers. Markers are biochemical or echographic parameters that appear in different values and frequencies in normal foetuses as opposed to foetuses with a given pathology.
A marker would be much more efficient when higher number of the affected foetus manifested it, constituting a diagnostic method only when the percentage is close to 100%.
- What are biochemical biomarkers?
Biochemical markers are substances produced by the foetus or the placenta that can be determined in the blood or urine of the mother.
The production of these substances may vary depending on whether the foetus is healthy or has certain pathology. Biochemical markers levels may also vary during the course of pregnancy, with some markers being only of any significant use if carried out during the first or second trimester. Marker levels can be expressed in different units and change with gestation time.
Because of this, and to obviate these difficulties and establish comparisons, apart from the usual units, biochemical markers are also quantified in MoM (multiples of the median). In general normal values for these markers usually range from 0.5 and 2.5 MoM.
- What are echographic markers?
Echographic markers are morphological foetal alterations detectable by ultrosund that cannot be classified as malformations. They are more common in foetuses affected by a given pathology than in normal foetuses.
Their presence increases the likelihood of the foetus being affected with aneuploidy and can appear at different times throughout the course of pregnancy and their presence may be short-lived.
- What types of prenatal screening are there?Several different screening modalities can be employed depending on the pathology to be detected, the time of pregnancy in which these modalities might be used and markers to be identified, as follows:
- Screening for chromosomal alterations
- Screening of neural tube defects
- Screening for cardiopathies
- Screening in multiple pregnancies
Screening for chromosomal alterations
This screening test is performed to assess the probability of a childbearing woman in an on-going pregnancy to carry a foetus with a chromosomal alteration. The most frequent and commonly assessed chromosome pathology by means of screening methods is trisomy 21, although trisomy 18 and 13 can also be determined. Depending on the markers used, Turner’s syndrome can also be detected (45, XO). The risk of a pregnant woman to be carrying a fetus with trisomy 21, 18 or 13 is related to her age. This refers to the so-called ‘a-priori’ risk for maternal age. Screening methods are based on a modification of the ‘a-priori risk’ for maternal age based on the results yielded by the combination of the markers analysed. If the analysed markers are normal, the final risk will decrease and if the markers are abnormal, such risk will increase. This probability or risk is calculated using computer programs that employ mathematical formulas published in the scientific literature. However, each program should have its own reference values mainly based on the laboratory techniques used.
Consequently, when different laboratories perform such tests, it is possible for the same pregnant woman, in an identical gestation, and with the same markers analysed, to obtain slightly different results when calculating the probability of foetus being affected with a chromosomal alteration or when calculating the risk. An established cut-off level in the screening tests is used to classify the screened patients into two groups. Such classification is usually expressed as a fraction of 1 amongst X (for instance 1:270), reflecting the way in which the cut-off level is applied whereby all patients below the 1:270 mark will run a higher risk of having an affected child (for instance 1:190) whilst all patients falling above the 1:270 mark will be considered low-risk (for instance 1:300). The perception of risk is very subjective and can differ greatly amongst different individuals. However, the aim of population screening programmes financed by the public health system is to attempt to achieve the maximum possible efficacy of the screening method used in order to obtain a cut-off point that optimises detection and that decreases the number of false positives. For standard screening tests, this cut-off point currently stands between 1:250 and 1:350. Different types of screening exist, depending on which markers are used and the time of the pregnancy when the screening is carried out.
First trimester
Biochemestry screening: this screening method analyses maternal blood between the 8 and 13 weeks of pregnancy and determines the values of certain substances, of which the most important are: B-hCGB-hCG (free beta fraction or total human chorionic gonadotropin) and PAPP-A (pregnancy associated plasma protein A). Nonetheless, other markers, which will probably be very useful during the first trimester of pregnancy, are currently under study. Screening based of two single markers is not commonly performed in the first trimester.
Echographic or ultrasound screening: this screening consists in the determination of echografic makers, of which the most commonly used at present during the first trimester are:
Nuchal translucency or nuchal fold scan (NT) is an echographic marker used in the first trimester (when you are from 8 to 14 weeks pregnant) that measures the thickness of the physiological liquid accumulated in the baby’s neck using ultrasound. The best time to do the scan is when the length from crown to rump (CRL) of the foetus is between 45 and 85 mm, which usually occur between the 11 and 14th weeks of gestation. Nuchal translucency thickness increases with the gestational age, and thus the importance of the measured thickness is in relation to foetal length. In general terms, a nuchal translucency measurement below 3 mm is considered normal in most cases.
Other echographic or ultrasound markers used include:
Assessment of the presence or absence of the nasal bone of the foetus. The face of individuals with Down syndrome is flattened and their nose is small. This might be attributed to a delay in the development of the nasal bone. Upon echographic exploration this echographic alteration manifests as the non-visualisation of the nasal bone in the first trimester or a smaller size than that expected for the second trimester. The nasal bone is absent in 60-70% of the foetuses affected with trisomy 21, in approximately 50% of foetuses with trisomy 18 and in 30% of foetuses with trisomy 13. The size of the nose is an ethnical feature and because of this in normal foetuses, the nasal bone might be absent in 1% of Caucasians, in 10% of Afro-Caribbean and in 7% of Asiatics.
 Presence of the nasal bone (please observe the two whitish structures making a = sign and the tip of the nose at the most prominent end)
Presence of the nasal bone (please observe the two whitish structures making a = sign and the tip of the nose at the most prominent end)
Absence of the nasal bone (a whitish structure which is the tip of nose is only observed)
The most commonly used echographic marker in the first trimester is nuchal translucency, whereas the rest of markers are usually used as second-line markers when nuchal translucency results reach borderline values or pregnancies at very high risk of chromosome pathology.
Ductus venous Doppler examination. The ductus venous is a vessel that transports blood from the placenta to the foetal heart; some sort of chromosomal alteration is suspected when the flow wave of this vessel is pathological.
Doppler assessment of tricuspid insufficiency: Tricuspid insufficiency occurs in 65% of foetuses with trisomy 21 and in 6% of foetuses who have healthy chromosomes.
Assessment of the craniomaxillofacial angle: An upper facial angle of 85º is present in 65% of cases of foetuses with trisomy 21 and in 5% of chromosomically normal foetuses.
Combined screening: This test consists in the combined use of the PAPP-A and free B-hCG markers (the most frequently used markers, although others can be added to the test) and echographic markers (the most common is nuchal translucency, although others can be added). This screening method achieves the best results in the first trimester.Second trimestre
Biochemestry screening: In this test, maternal blood is analysed between the 14rh and 20th week of gestation to determine the presence of certain biochemical markers. Depending on the marker used, the test will be referred as:
Double test: (alpha fetal protein) and B-hCG Triple test: AFP , B-hCG and uE3 (non-conjugated estriol) Quadruple test: AFP , B-hCG, uE3 and Inhibine-A At present it is standard procedure to assess two or three markers, although new markers are currently being studied that promise to be of great future utility.
Echographic screening: The different echographic markers described for the second trimester are used between the 18th and 22nd week of pregnancy, coinciding with the second morphological ultrasound (an ultrasound that studies foetal anatomy). Echographic markers differ greatly from one another and their significance for risk of chromosomal alteration is different for each of these markers. The most frequently used echographic markers include:
Nuchal fold assessment, which consists in the measurement of the thickness of the foetal neck between 18 and 22 weeks. Generally, a nuchal fold below 6 mm between the mentioned 18 and 22 weeks is normal in most cases; however, when the nuchal measures over 6 mm, it is indicative of increased risk of Down syndrome.
Cysts in the choroid plexuses may be present in different number and size along the thickness of choroid plexuses, which are structures located in the cerebral ventricles. Their presence increases the risk for trisomy 18, although they are present in about 2% of normal foetuses and disappear totally between the 25th and 28th week of pregnancy. At present there is wide consensus on the advantages of combining the information provided by echographic and biochemical markers.Sequential screening
This modality of screening modifies risk as the results of new explorations become available. For instance, in a childbearing woman who had a combined screening in the first trimester with a risk of 1:1000, the presence of echographic markers for chromosomopathology in the morphological ultrasound will increase the risk in 1:200 or more, whereas the absence of echographic markers in the ultrasound will decrease the risk to 1:5000 or less.
Let’s see:
Integrated screening: This test takes into consideration the PAPP-A and Beta-hCG values obtained during the first trimester of pregnancy and the values obtained during the second trimester.
To increase the reliability of this test, other results are considered as the ultrasound results of the nuchal translucency, performed during the first trimester of pregnancy, and the nuchal fold results performed during the second trimester of pregnancy.
Other ultrasound markers, called “soft markers” such as the length of the femur, humerus, hypoplasia of the mild phalange of the small digit, absence or hypoplasia of nasal bone, measurement of iliac bone, dilated renal pelvis, cardiac defects, etc.
The combination of the results obtained through the biochemical screening and the ultrasound examinations allows the clinician to recalculate the risks depending on the maternal age, race, weight , absence or presence of insulin-dependent diabetes, number of children (twins, triplets). The pregnancy will be considered to be at risk when the risks exceed a certain threshold (or cut-off point), which is generally estimated at 1 in 250. Sensitivity is high, standing at about 90%, with a 3% rate of false positive, the results are not reported until all the tests have been done, which is approximately during the 16th week of pregnancy.
In cases where the expecting mother starts a late consultation in the office and it is not possible to perform the ultrasound examinations or biochemical screening tests of the first trimester, it is possible to still diagnose abnormalities in about 85-90% of cases by combining the ultrasound markers of the first trimester + the biochemical screening tests of the second trimester.
Screening of neural tube defects
Biochemical screening
This test determines AFP values in maternal blood. This blood test must be performed in the second trimester between 15 and 18 weeks and not before the 15th or after the 20th week. There is risk for the existence of neural tube defects if AFP values are over 2-5 MoM. AFP levels can also be determined in the amniotic fluid. Risk exists when the amniotic fluid is above 2 MoM. Some situations such as vaginal bleeding can also increase AFP values, a situation which invalidates it as a screening method.
Echographic screening
This screening method is used to visualise the integrity of the vertebral column and the normality of intracranial structures in the morphological ultrasound. Currently some centres that perform combined screening tests in the first trimester and are equipped with a high-resolution ultrasonography do not deem necessary to screen neural defects by biochemical screening, because they considered echographic screening to be sufficient.
In summary: AFP values are used to detect possible defects of the neural tube, such as open spina bifida and anencephalia.
Whenever increased values of alpha-fetoprotein are detected, an ultrasound examination should be performed to determine the exact gestational age, a diagnosis of multiple pregnancy or to detect fetal-placental structural defects. According to the results, amniocentesis may be required.
If AFP levels in maternal blood are higher than 2.5 MoM or higher than 2 MoM in amniotic fluid , it is possible to measure acetylcholinesterase levels in amniotic liquid as a diagnostic test to screen for neural tube defects. Please remember, the attainment of a pathological result when maternal screening tests are done is only an indication ON THE POSSIBILITY of some abnormality, but it DOES NOT confirm its existence.
Screening for cardiopathies
Measurement of nuchal translucency thickness (measurement of the fetal neck thickness) is also a good marker of cardiopathy. In foetuses that present a nuchal translucency above 3mm and a normal kariotype (obtained by invasive means), it is advisable to perform a foetal echocardiography to rule out the presence of a cardiac malformation. The presence of an abnormal flow wave pattern from the ductus venous also increases the risk of cardiac malformation.
Screening in multiple pregnancies
In twin pregnancies it is possible to use either the biochemical screening or the echographic screening, whereas in pregnancies of more than 2 foetuses only the echographic screening is normally used.
Most computer programs are designed to calculate the risk in twin pregnancies. The use of biochemical markers on their own determines the risk of the pregnancy itself (not the risk for each foetus), whereas echographic markers, or the combination of both, assess the specific risk for each foetus.
In summary: the fiability of the biochemical test using the mother’s blood it is lower because all fetuses contribute towards the final concentration of the substances measured using the mother´s blood, which could lead us to wrong interpretations.
- What is the expected effectiveness of the different screening methods?The effectiveness of a screening test is based on its capacity to detect truly healthy individuals and the percentage of false positives that the test yields (healthy individuals found to be affected by the test). If the percentage of false positives remains unchanged (generally up to a 5% is found to be acceptable), the comparison of the different screening methods can be based exclusively on their detection capacity. Please below find an orientative table showing the expected detection capacity of the different screening methods for a 5% percentage of false positives.
Type of screening Detection capacity Biochemestry in the first trimester 60% Ultrasound in the first trimester (NT) 75% Ultrasound in the first trimester (NT) 85% Biochemestry in the 2nd trimester (2 markers) 60% Biochemestry in the 2nd trimester (3 markers) 65% Biochemestry in the 2nd trimester (4 markers) 70% Some recent studies inform us about the possibility of the screening for trisomy 21 in the first trimester (which combines ultrasound for nuchal translucency and nasal bone + the determination of free Beta-hCG and PAPP-A in maternal blood) allows the clinician to potentially identify over 90% of pregnancies with this syndrom, with a false positive rate of 2,5%. For further information try logging onto The Fetal Medicine Foundation: The Fetal Medicine Foundation
In relation to the detection capacity of the combined screening method in the second trimester, the data available at present are scanty.
The use of this techniques requires:
- Highly qualified professionals in this field.
- The use of devices and laboratories equipped with the latest technology and approved by the competent regulatory authorities.
- How to interpret the results I have obtained from the biochemical screening - RISK CALCULATOR
Access the risk calculator specifically created so you can corroborate the information you have received regarding the risk that your pregnancy may present trisomy. Enter your data in the corresponding boxes, follow the instructions provided and carefully read the complementary information for a correct interpretation of results
Access the trisomy risk calculator
Additional information that may be useful to you
How can I assess the results obtained in the screening of Down’s syndrome?
In order to assess the results you have obtained, it is important that you first read all the information provided in this section (prenatal diagnostic techniques) and based on this information you take the following guidelines into consideration:
1.- The risk that a foetus may carry a chromosomal anomaly is calculated on the basis of the risk that the pregnant woman presents due to her age at the moment of delivery modified by the result obtained with biochemical and/or ultrasound markers.
This table indicates the risk of trisomy 21 at the moment of delivery based EXCLUSIVELY on the mother’s age.
Mother’s Age Risk of T 21 at delivery 18 1 in 1562 20 1 in 1538 25 1 in 1380 30 1 in 966 35 1 in 428 38 1 in 215 40 1 in 129 42 1 in 75 44 1 in 43 2.- The values of the biochemical or ultrasound markers vary in the different weeks of gestation. In order to compare results and assess them separately from gestation length, when calculating risk, these value are expressed in MoM (multiples of the median). If your blood test is not expressed in MoM, ask your laboratory to perform the conversion.
3.- Overall, the normal value for different markers is 1 MoM. Therefore, the farther away from 1 MoM the marker’s result is, the worse the result is. This table shows reference values, expressed in MoM. A marker is considered suspicious if its values are greater (>) or lower (<) than these reference values.
Marker MoM TN >1,8 – 2 PAPP-A < 0,4 Beta hCG libre o hCG <0,4 o >2,5 AFP <0,4 o >2,5 uE3 < 0,5 Inhibina A > 2,5 NT (nuchal translucency)
This is the most important ultrasound marker and the most influential on risk calculation. No pathologies associated with a NT below 1 MoM are known. The thicker the NT is, the worse foetal prognosis is. Pathological values of NT usually range between 1.8 – 2 MoM or a measure greater than 3 mm (regardless of the MoM).
Please note, do not confuse “nuchal translucency” with “nuchal fold”, as these are two different ultrasound markers.
PAPP-A. (pregnancy-associated plasma protein A)
Overall, foetuses with chromosomal abnormalities have low PAPP-A values. Values lower than 0.4 MoM increase the risk of chromosomal anomaly.
Free beta hCG or hCG (total human chorionic gonadotropin or its free beta fraction)
Foetuses with chromosomal abnormalities can present altered free beta hCG or hCG values. Overall, in Trisomy 21 these values are higher, those higher than 2.5 MoM indicating a possible pathology. In Trisomies 13 and 18, these values are generally low, with suspicious values being those below 0.4 MoM.
AFP (alpha-fetoprotein))
High levels of AFP, higher than 2.5 MoM, may indicate foetal malformation (spina bifida, anencephaly, etc.). Low levels of AFP, lower than 0.4 MoM, are observed in certain chromosomopathies. Hence, in trisomies 21 and 18 AFP may present low values whereas in trisomy 13 it can be slightly higher than normal.
uE3 (unconjugated estriol)
Values lower than 0.5 MoM can indicate possible chromosomopathy of chromosomes 21, 13 or 18.
Inhibina A When its values are greater than 2.5 MoM, they may be indicative of trisomy 21 or 13.
Remember:
These are reference values, but in order to calculate the probability of chromosomal anomaly or risk the different markers expressed in MoM must always be combined with the risk for the mother’s age.
That these markers are part of DETECTION tests and not DIAGNOSTIC tests, so even if a value or marker appears to be pathological, it does NOT mean that a chromosomal abnormality has been diagnosed, given that these tests are NOT diagnostic. These tests only INFORM US ON THE POSSIBILITY that there may be some type of risk. Hence, once you have received your results, these must be assessed by your physician to determine if it is necessary to perform other complementary tests (chorial biopsy, amniocentesis, etc.), given that he has your complete clinical history to aid him in adequately interpreting and correlating these data. Thus, if you are feeling anxious or if there is some information you do not understand or that is concerning you, try to CALM DOWN and ask for an earlier appointment with your physician. Let us insist on the fact that these are not diagnostic tests, but rather detection or tests to rule out any suspected anomaly.
NUANCES of these types of tests
4.- Why can I have different levels of Risk if screening is carried out in two different laboratories in a short period of time (1 week).
Because the computer program used to calculate risk is not the same in both centres or biochemical markers are not identical.. Each computer program, used to calculate risk, has reference values (population parameters, etc.) and each marker has medians (used to calculate MoMs) that may vary and that influence risk results, causing these differences. If the difference were significant, we would have to contemplate the possibility that one of the two screening systems had not been correctly configured. In cases of discrepancy, screening must not be repeated to “check” results given that they are statistical estimations and not true diagnoses. If an error is suspected, talk to your doctor or with the laboratory head.
5.- Are these results equally reliable in the case of twins or triplets? Can the fact of these twins or triplets being monozygotes or hetozygotes affect the results?
These results are very reliable in the case of twins (mainly if they are monozygotes, in other ways, identical but not of much use in the case of triplets. In heterozygotic twins (different to each other), the combined screening of the first trimester (which includes NT) is much better than the screenings that only include analytical parameters and no ultrasound parameters.
6.- Are these results equally reliable if it is a twin gestation at the beginning and afterwards one of the twins stops growing and developing (evanescent twin)?
Increased levels of human chorionic gonadotropin (hCG and free beta hCG) have been described, specially during the first trimester of pregnancy, due to the presence of an evanescent twin. In theory, the presence of an evanescent twin can alter alpha-protein levels in maternal serum but not in the amniotic fluid, as these are specific to each amniotic sac and normally the evanescent twin and the living twin are inside different amniotic sacs. The presence of both foetuses inside the same amniotic sac, even though possible, is not a common occurrence and it poses a very high risk situation in which each case should be assessed individually.
7.- Can the values of these markers be altered if I am a diabetic or I inject myself with insulin?
When insulin-dependent diabetes is not properly controlled, it may sparingly alter the values of biochemical markers (those found in the mother’s blood), but if the person responsible for carrying out the screening is informed of this situation, a correction will be made to compensate for such circumstance.
8.- Can the consumption of alcohol or smoking alter the results of these tests?
The results from the biochemical markers used for the screening of trisomies change depending of the number of cigarettes that the pregnant women smokes. This situation should be reported to the doctor, so that, as in the case above, the necessary correction is made to compensate for such circumstance. No changes have been reported with regard to the consumption of alcohol.
9.- What other causes may change these results?
The pregnant woman’s weight and certain racial traits (black, Asiatic ethnicity, etc.) are among the main causes that have the potential to change biochemical markers. Because of this, most screening centres ask pregnant women these and other type of questions in their information sheets so that the necessary compensatory corrections are made to improve the screening results.
10.- Today I had my 12th week ultrasound done. I am very concerned because, even though at the beginning everything was OK, the test recommends that I have an amniocentesis done. I do not understand this and I get very little information, should I be worried?
This means that when the ultrasound was done, you were told that it was normal but subsequent blood tests have shown slightly altered values (beta free hCG, PAPP-A test, etc.). These results might indicate a risk situation and this is why in such cases an amniocentesis is recommended. You must trust your healthcare professional and follow his or her instructions. For your peace of mind, you should know that it is important that your first ultrasound was normal. In other words, if with the same risk results the first ultrasound you had done had been abnormal, the possibility for the amniocentesis to be abnormal would also be higher.
11.- How do I interpret the percentages I am being given?
The results of a screening test are expressed as a probability of “1 in a given number” for instance, a 1 in 50 probability means that if a mother were to have 50 pregnancies, the foetus in 1 of these 50 pregnancies would be affected (the foetus would have Down syndrome if the screening had been for that specific chromosopathy). If this probability were to be expressed as a percentage (number per cent), then it would be 1 * 100/50 = 2% which is the same and represents a 2% probability of having a child affected with Down’s syndrome.
Risk perception is very subjective and personal. It is generally considered that any risk greater than 1 in 250-350 (cut-off level) is sufficiently high to recommend a confirmatory invasive technique (amniocentesis, chorion biopsy etc.). Please remember, the lower the number appearing to the right of the risk fraction the greater the risk is; thus a risk of 1 in 50 is greater than a risk of 1 in 100 and much greater than 1 in 1000. 1 in 1000 is usually expressed as 1:1000.
As previously explained, to calculate the risk of a pregnant woman having a foetus affected with trisomy 21, 18 or 13, first the mother’s risk based on her age at the time of delivery has to be calculated. Next, the resulting figure (risk) is modified and recalculated based on the information yielded by the different markers of screening method being used.
Let’s see an example:
Let’s suppose a first trimester combined screening is performed on a women that will be 43.5 years old on the estimated due date who has a risk adjusted by her age of 1 in 50.
The screening has been completed and the result yielded by each marker (beta hCG, PAPP-A and TN) has been 1 MoM. The risk result for trisomy would be 1 in 824 ; that is, much lower than that corresponding to her age and given that the risk is lower than 1 in 250 (cut-off value for the indication of amniocentesis at her healthcare centre), amniocentesis will not be recommended.
If on the contrary, this same pregnant woman had 2 MoM beta hCG, 0.5 PAPP-A and 1.5 MoM TN she would have the same risk for her age, that is 1 in 50; however, since the ultrasound/biochemical markers were somewhat altered, the risk for trisomy 21 would change from 1 in 50 to 1 in 13, and therefore amniocentesis would be rendered appropriate.
An invasive technique (amniocentesis, chorion biopsy, etc.) should be recommended whenever the risk for trisomy 21 or for trisomy 18 or 13 is higher than the cut-off point established at each centre (1 in 250-350) but should never recommended in cases where the risk for trisomy (21 or 18-13) is greater than the risk for the pregnant woman’s age.
12.- If the first trimester screening was normal, is it necessary to have the second trimester screening?
No. It is not necessary as the first trimester screening is much more reliable than the second trimester screening.
Given that:
the 1st trimester screening is based on the mother’s age, an ultrasound parameter, and two biochemical parameters, it allows us to detect between 80-85% of cases of Down syndrome, with a 5% rate of false positives (no risk-free results and normal fetus). The 2nd trimester screening is based on the mother’s age and on two biochemical parameters. This second screening allows us to detect between 60-65% of cases of Down syndrome, with a 7-10% rate of false positives.
It is only in the so called “Integrated Test” that combines markers from the first and second trimesters that determinations are carried out in both trimesters, but the result is only obtained after the second trimester blood tests are done. The so called “contingent screenings” are under study and, in some centres they are performed as research. In these screening tests, women are classified into three groups according to the risk score obtained in the combined screening test performed during the first trimester: low risk, high risk and intermediate risk (borderline). This latter group is advised to undergo a screening test in the second trimester the Final Risk of which is obtained by combining the results of the first trimester with the results of the second trimester, as in the Integrated Test.
13.- If the PAPP-A value is low and the beta hCG normal, could it possibly indicate any anomalies in sexual chromosomes?
Some Turner’s syndromes present a low PAPP-A with a normal hCG.
14.- Should the ultrasound study (nuchal translucency) and the maternal serum screening be performed on the same day?
No, it is not necessary to have both tests on the same day. Whether they coincide or not will not alter the final result, for as long as these studies are carried out within the time period or gestational weeks as indicated by the obstetrician.
15.- Must the risk be recalculated if the mother’s age is incorrect?
Yes. If mother’s age is incorrect it must be modified by the real one (ideally her age at the time of delivery with a decimal point that will correspond to the decimal fraction of the months) and the risk should be recalculated once more, given than the mother’s age is a very important marker (the most important factor that we have).
16.- Must risk be recalculated if the gestational age is incorrect?
Yes. Gestational age is used to calculate the biochemical markers MoMs and if the gestational age is incorrect also will be incorrect the results obtained in biochemistry MoMs.
17.- What does “risk adjustment” mean in the case of triplets?
It means that some computer programmes calculate the risk for triplets “(by dividing the MoMs by 3)”. Nowadays, this test is COMPLETELY UNRECOMMENDED. At the most, individual risk may be calculated for each of the triplets by means of echographic markers (NT – and CRL – crown-rump length) only, but never by current biochemical markers.
18.- Is it true that beta-hCG is higher in twin gestations?
Yes, beta-hCG values are nearly two-fold those reported in single pregnancies, as with the majority of biochemical markers.
- What is the difference between biochemical screening and free-fetal DNA screening?
Biochemical screening measures the concentrations of biochemical markers in maternal blood from the placenta and fetus.
Free-fetal DNA screening measures specific genomic sequences of circulating free-fetal DNA from placental cells in maternal blood.
Neither of the two screenings are diagnostic tests, for neither of them invades the fetal space. Both are probabilistic population screening tests, with false positives and false negatives, performed from a maternal blood sample.
- Which screening is easiest, biochemical screening or free-fetal DNA screening?
Free-fetal DNA screening can detect 99% of fetuses with trisomy 21, 97% of fetuses with trisomy 18, and 92% of fetuses with trisomy 13. With a false positive rate of 0.1%,0,2%, 0,2%. This screening should be done in combination with a SPECIFIC ultrasound study at 10 and 13 weeks gestation to correctly determine gestational age, number of embryos implanted and, if more than one, assess their chorionicity and look for suggestible specific chromosomal fetal morphological abnormalities.
Please check the following links for further information:
Taking the Harmony Prenatal Test
The Fetal Medicine CentreDepending on the markers that are used, the detection rate of biochemical screening ranges between 60 and 85% with a false positive rate of 6%. When SPECIFIC ultrasound examination at 10 and 13 weeks of gestation is added to identify morphological alterations suggestible of specific fetal chromosomal, the test allows a detection rate of 95% of pregnancies with trisomy 21, with a false positive rate of 2.5.
You will find further information at:
The Fetal Medicine Centre
Hospital Universitario Virgen de las Nieves – Aneuploidy screening in the first trimesterBoth tests must be performed in highly specialized centres by qualified and trained professionals in this field.
Both screenings must be performed in combination with ultrasound examinations at 10 and 13 weeks gestation to rule out malformations or ultrasound markers suggestive of chromosomal abnormalities to correctly determine gestational age and assess the number of implanted embryos, given that both tests (screenings + ultrasound examinations) complement each other.
Besides, in normal circumstances, all pregnancies should be monitored by regular ultrasound examinations at 20-22 + 30-32 weeks of gestation to monitor the anatomy, growth and well-being of the fetus.
If during the course of a pregnancy, a problem or malformation is detected that requires further ultrasound monitoring during pregnancy, the protocol to be followed will be individualized and as established jointly by the ultrasonographist and the OB-GYN.
- Why is free-fetal DNA screening not performed in all patients?
Because of the economic cost. Currently free-fetal DNA screening is much more expensive than biochemical screening. In addition, the results are much faster with the biochemical test than with the free-fetal DNA test (10-14 days).
- Why is there so much interest in screening tests?
1.- Because they are easy and simple to perform.
2.- The test involves extracting 20 ml of blood from the mother, and as you can guess this does not pose any risk of miscarriage, or any harm to the fetus, allowing us to obtain valuable information during the first trimester of pregnancy.
3.- Because screening tests are much cheaper than diagnostic tests and allow us to perform routine scans of the most frequent chromosomal abnormalities that are associated with chromosome 13, 18, 21, X, Y on the general population.
4.-Because after the human genome-sequencing project and the great advances that are taking place in this field, new biotechnology platforms are continuously emerging. Some laboratories are offering the diagnostic possibility of using the same sample of maternal blood to perform studies to detect trisomy + triploidies + the most common microdeletions: 22q11.2 (Di George syndrome), 1p36, Angelman syndrome, Prader Willy syndrome and Cri du chat syndrome. All these diseases, besides malformations, involve mental retardation.
- Can biochemical screening and free-fetal DNA screening be performed at the same time?
It makes no sense if any of the two has been performed and the results have been normal.
If in the absence of ultrasound abnormalities, the first test, that is biochemical screening, is performed and it is positive, before performing an invasive test that involves some risk of miscarriage, the free-fetal DNA screening test should be performed and if this test is also positive for some pathology, then it is recommended that an invasive test (chorionic villus sampling, amniocentesis, cordocentesis) be performed to rule out these or other chromosomal abnormalities not detected by this type of test.
If both tests (biochemical and free-fetal DNA) have been normal, but on ultrasound fetal malformations are observed, an invasive test (chorionic villus sampling, amniocentesis, cordocentesis) should be performed to rule out other chromosomal abnormalities that are not related to chromosomes that were studied in the screening.
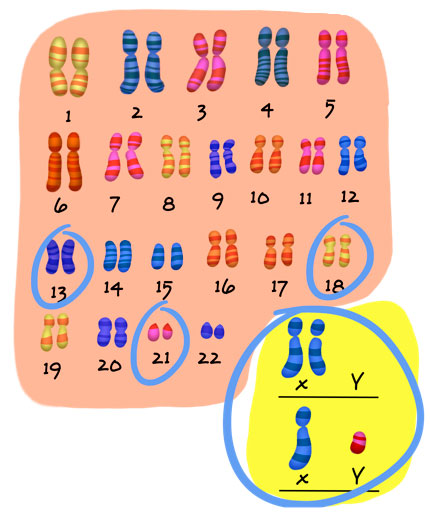
Remember that the human species has 23 pairs of chromosomes and that the chromosomes that are routinely studied with these techniques are: 13, 18, 21, optionally the X, Y chromosomes, and in some cases, microdeletions of some of them.
- What do these tests detect?
Biochemical screening usually tests for trisomies 13, 18, 21, and Turner syndrome (45, X) – an individual who lacks a sex chromosome- , or certain malformations including neural tube defects such as spina bifida or anencephaly.
In the free-fetal DNA screening test, laboratories offer different diagnostic possibilities that can include the diagnosis of:
1. Trisomies of chromosomes 13, 18, 21.
2. The study of the five chromosomes together 13, 18, 21, X, Y.
3. The study of chromosomal alterations related to chromosomes 13, 18, 21, X, Y, + triploidies + microdeletions of: 22q11.2 (DiGeorge syndrome), 1p36, Angelman syndrome, Prader Willy syndrome and the cri du chat syndrome. - What are the disadvantages of these tests?
1.- The disadvantage is that they do not analyze the 23 chromosomes we have, but only a part of them. Nevertheless, it must be said that the chromosomes analyzed are those most frequently abnormal. However, it is worth remembering that any chromosome can be abnormal in number or structure and lead to various diseases and if they are not studied, diseases cannot be diagnosed. Moreover, if when the ultrasounds are performed, no malformations are observed, these pathological conditions cannot be suspected.
2.- That they are NOT diagnostic tests but screening tests, which means that the results are expressed as the PROBABILITY or likelihood that a fetus has a high or low risk for having Down syndrome (trisomy 21), Edwards syndrome (trisomy 18) or Patau syndrome (trisomy 13), a problem of sex chromosomes or microdeletion. Everything depends on the type of study performed. Consequently, these tests are not exclusive; the low or high probability still exists, although screenings performed using free-fetal DNA, reliability is very.
3.- As probabilistic tests, they can yield false positives and false negatives.
A false positive is when testing indicates that the fetus probably has one of the chromosomal abnormalities studied, when, in fact, it does NOT have it.
And, a false negative is when testing indicates that the fetus probably does not have the chromosomal abnormality, when, in fact, it DOES have it.
Hence the great importance of performing the ultrasound examinations at the same time that these tests are performed, since many of these chromosomal abnormalities are accompanied by signs or transient changes in fetal anatomy that are suggestive of these abnormalities, during very specific gestational periods.
- What if there are twins or triplets in the gestation?
In BIOCHEMICAL screenings, detection rates can vary, hence the importance to perform the screening at the same as the ultrasound examinations at 10 and 13 weeks of gestation in order to change correction rates appropriately and interpret the results correctly.
These results are quite reliable in the case of twins (especially if they are monozygotic, ie identical) but not that useful in triplets. In heterozygous twins (different) the combined first trimester screening (including nuchal translucency) is much better than the screenings that only include analytical parameters and no ultrasound parameters. In the event that one twin stops developing (vanishing twin) increases of human chorionic gonadotropin (hCG and free beta hCG) have been reported, especially in the first trimester of pregnancy.
Free-fetal DNA screening is feasible in the case of twins.
- What is maternal blood screening?
This is a biochemical test using the mother’s blood during a few specific weeks of pregnancy. Maternal serum screening is used to identify those pregnant women who are at risk of having a baby with some chromosomal alteration or certain malformations.
Given the great importance of this type of tests, we recommend that you read carefully the information in the section “Prenatal diagnosis techniques” as you will find this overall reading of great help.
Please remember, the attainment of a pathological result when maternal screening tests are done is only an indication ON THE POSSIBILITY of some abnormality, but it DOES NOT confirm its existence.
For further information: The Fetal Medicine Foundation
- How many ultrasounds should be performed during pregnancy?Habitually, three ultrasounds are routinely performed.
- The first ultrasound is performed during the first trimester of pregnancy to verify that it is a uterine pregnancy and to determine the number of embryos, and their viability. Because ultrasound monitors foetal development, it may also be able to identify suspected chromosomal alterations that will have to be reconfirmed by the previously described diagnostic procedures (chorion biopsy, amniocentesis, etc.).
- The second ultrasound is performed during the second trimester of pregnancy to rule out any foetal malformations.
- The third ultrasound is performed during the last trimester to assess the growth and development of the foetus and to verify its condition in preparation for the actual birth.
In addition, an obstetrician or echographist may also require an ultrasound in cases where specific echographic monitoring is indicated.
- Is it dangerous for the foetus to be exposed to several ultrasound examinations during pregnancy?
NO, it is not dangerous. However, only those ultrasounds that are strictly necessary should be performed. An ultrasound is not a toy that should be played with. It is a diagnostic working tool.
- Which are the risks of having a prenatal X-ray exposure for the unborn child in a pregnant woman?
To be able to assess the risk of causing damage at an embryo-foetal level due to radiation exposure, we should take into account the week of gestation and the dose of radiation received.
During the first two weeks of pregnancy, the law of all or nothing applies, that is, nothing happens or the embryo dies, putting an end to the pregnancy.
From the second week until the sixth week, a wide range of malformations can develop based on the radiation doses to which the embryo might have been exposed.
This is because we are at the critical period where the baby’s future organs are being developed.
The possible teratogenic effects may include prenatal-onset growth retardation, central nervous system damage, including microcephaly and ocular anomalies. Radiations (absorbed doses) are measured in Rad (quantity of energy deposited per kg in the tissue. An approximate estimation of the foetal irradiation in any different diagnostic X-Ray examination performed in a pregnant women was calculated:
- Thus, for instance, it is known that in a chest X-ray the exposure is very low, in the order of 0.07 mrad; in a lumbosacral X-roentgenolography is in the range of 0.300 rad; and in an endovenous pyelography, the dose can exceed 1 rad.
- In explorations with contrast, the foetal exposure differs according to the type of study. Brain angiography is <10mrad, an enema from 1945 to 3986 mrad.
- A CT scanning of the lumbar column before the 11th week of gestation , the irradiation will be 3 rad and with a brain CT scanning, the minimal dose will be 0.05 rad.
Doses higher than 25 rad, an embryopathology is certain if the irradiation takes place during or before the critical period, and if received afterwards, it will certainly lead to foetal malformations.
Doses over 100 rad will certainly cause damage to the unborn child at any stage of the pregnancy.
We may conclude that radiographic exposure should be avoided unless there is an expected important benefit.
- If the chromosomal study comes out normal, does it mean our child will be normal?
No, because the unborn child may have a disorder that may not be chromosome-related, or a malformation of a different origin (infection, etc.).
- What is nuchal translucency?
Nuchal translucency or nuchal oedema is an ultrasound marker of the first trimester (8-14 weeks of pregnancy), that consists in the measurement of the physiologic liquid accumulated in the baby’s neck via ultrasound.
Nuchal translucency increases physiologically with the gestational age, and can range between 2.4 mm at 12 weeks of pregnancy, to 2.9 mm at 14 weeks.
At present, the measurement of nuchal translucency has become the most sensitive and easy to obtain marker for the detection of some fetal pathologies, being present virtually in all foetuses between weeks 8 and 14 of pregnancy.
The space is measured from the external surface of the cervical column to the internal part of the cutaneous area, appearing upon ultrasound as a well circumscribed echonegative area.
If you require specific information on the position the foetus must be in at the time of the measurement, this link can be of help.
What is the best time to perform this test?
The nuchal translucency test can be performed from the 8th to the 14th week of pregnancy. After the 8th week by jeans of 3D vaginal ultrasound (three dimensional), but the ideal time to detect any abnormalities is between week 11 and week 13+6 days of pregnancy by jeans of 2D (bi-dimensional) vaginal or abdominal ultrasound, or 3D.
As a general rule, nuchal translucency is a transitory marker that disappears after the 14th week.
However, be careful with this information! Abnormal results can persist physiologically in about 2% to 4% of normal foetuses due to delayed closure of the upper part of the vertebral column or may also be pathological in cases of chromosomal abnormalities, malformative syndromes or other conditions that are not related to chromosomal abnormalities.
When is nuchal translucency considered normal?
Nuchal translucency is considered physiological when:
- Its thickness is less than 3 mm
- It is homogenous and lineal
- It is homogenous and lineal
When is nuchal translucency considered pathological?
It is suspected to be pathological when:
- Its thickness if over 3 mm.
- It is not lineal and small septa or irregularities are observed.
- The translucent space has a variable refringence.
- The atlas and axis (bones of the upper part of the vertebral column) remain open after the 12 week of pregnancy.
- When bullae or cystic structures are observed.
- Translucency extends beyond the cephalic pole or fetal back.
- When it is accompanied by ascitis or fetal anarsarca
- When other accompanying abnormalities are detected.No es lineal y se observan pequeños septos e irregularidades
For further information:
Book: Ultrasonidos 3D-4D en Obstetricia.
Authors: F. Bonilla-Musoles, L.E. Machado
Medical Publishers, panamericana, 2005.What can be done when the nuchal translucency test is positive?
The most important thing is NOT to get alarmed and to follow your doctor´s orders. When nuchal translucency is assessed, your doctor will take into account other biochemical studies performed during the first trimester and will subsequently give you guidelines that you will have to follow, among these:
- Ultrasound follow up of the foetus
- A study of fetal chromosomes by jeans of a chorionic biopsy or amniocentesis
How should we evaluate a positive nuchal translucency?
The risks for chromosomal abnormalities are calculated taking into account the ultrasound findings and the biochemical screening results obtained from the maternal blood.
For a nuchal translucency of 4 mm, the risk of chromosomal abnormalities is approximatelly 20%; for a nuchal translucency of 5 mm, the risk is 33%; for a nuchal translucency of 6 mm, the risk is 50%, and for a nuchal translucency greater than or equal to 6,5 mm, the risk is 65%.
PThus, the first step in the management of these pregnancies is to perform a fetal chromosomal assessment via a chorion biopsy.
For further information, log onto The Fetal Medicine Foundation.
Nevertheless, let’s NOT forget that certain abnormalities observed during the first trimester can correspond, in some cases, SIMPLY to transitory physiological or pathological situations that disappear leaving no evident sequelae in the newborns,
BECAUSE OF THIS, REMEMBER THAT AN ABNORMAL NUCHAL TRANSLUCENCY RESULTS IS ONLY INDICATING THE POSSIBILITY OF A GREATER RISK FOR SOME DEFECT and that subsequent studies will confirm or rule out such hypothesis.
Why is it necessary to continue having ultrasound examinations after a positive nuchal translucency and a normal chromosomal study?
- Because although most foetuses that present with increased nuchal translucency and a normal karyotype will be normal babies, it is advisable to exclude in these cases abnormalities such as cardiac defects, diaphragmatic hernia, skeletal dysplasias, renal defects, obstructive renal disease, onphalocele, as well as some other rare genetic syndromes (Noonan, Smith-Lemli-Opitz, Stickler Artrogriposis or multiple pterigium syndrome, etc.).
- PBecause 30% of translucencies greater than 5 mm are associated with disorders linked to higher perinatal morbimortality that are not related with chromosomal abnormalities. The aim of these ultrasound examinations is to assess the foetal wellness.
For that reason, in cases of increased nuchal translucency > 3mm and normal foetal karyotype, a detailed scan, including a foetal echocardiography (a study of the heart) should be performed between the 14th and 16th week of pregnancy to ascertain the progression of the nuchal translucency and to exclude or diagnose multiple foetal abnormalities. A detailed ultrasound examination should also be performed at 20-22 weeks to either exclude or diagnose more subtle malformations or defects associated with genetic abnormalities. If everything is in order, the risk to give birth to a newborn with a severe abnormality or psychomotor retardation is not higher than that of the general population.
What is the nuchal skin fold?
This is an ultrasound marker used in the second trimester of pregnancy consisting in the measurement of the thickness of the subcutaneous tissue at the level of the baby’s neck. This test should be performed between the 14th and 22nd weeks of pregnancy and it is considered pathological when it is above 6 mm.
In foetuses with Down’s syndrome (trisomy 21), this value is increased.
Please note, do not confuse “nuchal translucency” with “nuchal fold”, as these are two different ultrasound markers.
For further information:
The Fetal Medicine Foundation.
Book: Ultrasonidos 3D-4D en Obstetricia.
Authors: F. Bonilla-Musoles, L.E. Machado
Medical Publishers, panamericana, 2005. - Fetal or perinatal infections, how can viruses and bacteria affect my pregnancy?
There are many types of viruses or bacteria that can produce fetal or neonatal infection via the infected mother.
There are several transmission routes: transplacental (via the placenta), through the birth canal and breastfeeding.
The effects on the fetus or newborn are different depending on:
- The type of virus or bacteria that produces the infection.
- The type of maternal infection: first time the mother acquires the infection
- Re-activation
- Active chronic infection
- Re-infection
- The time at which the fetus becomes infected (first, second or third trimester of pregnancy), or peripartum period
- The tropism of the virus or bacterium for different organs, for example, parvovirus B-19 has great affinity for the hematopoietic system, while cytomegaloviruses have affinity for the central nervous system, etc.
- Citomegalovirus
- Rubeola
- Parvovirus B-19
The viruses that produce fetal-neonatal primarily in the peripartum period are:
- Herpes simplex
- Hepatitis B
- Hepatitis C
- Human Immunodeficency virus, HIV-1 AND HIV-2 (AIDS)
For more information on these infections, click on the its name:
For more information visit March of Dimes.
Remember, if you are planning on getting pregnant it is very important that you:
- Before becoming pregnant, see your gynecologist to make sure everything is okey and to rule out any infectious processes using the appropriate tests. This will make it possible to assess whether you present any special risk that should be taken into account and the possibility of administering some type of treatment in order to reduce the risk of transmission to your child.
- During pregnancy, it is very important that you continue with the pregnancy follow-up indicated by your OB/GYN based on your age, reproductive medical history and other complications that could arise during pregnancy.
Remember:
- There are specific diagnostic methods for each type of infection. The correct interpretation of the results obtained should only be performed by your doctor, as he or she has your complete clinical history and will know how to correlate all information, while assessing whether it is necessary to repeat or run further tests to complete your evaluation and determine whether it is a past or recent infection, given that infection levels may vary. It would be the same scenario as a person who presents with a limp. If we photograph him we will not notice the limp, but if we film him we will. If we apply this same concept to infections we can see that in some cases, one test is insufficient to reach a final diagnosis, making it necessary to perform additional tests to see the evolution of the biochemical parameters that are being measured and finally to reach a diagnosis.
- If you are pregnant and have been exposed to chicken pox or any other infection, contact your doctor immediately in order to assess whether your child is at risk and what preventive measures should be adopted.
- Is it possible to perform surgery on the foetus?
It has been attempted to treat some malformations, but it is still at an experimental stage.
- What is gonadal mosaicism?
Mosaicism is defined as the presence of two or more cell lines. These cell lines may have originated at the expense of anomalous repartitions during cell divisions subsequent to fertilisation.
- If these errors occur in organs involved in reproduction, that is in the ovary or testis, the mosaics are called gonadal mosaicism. These errors can either lead to normal or pathological gametes. If a pathological gamete succeeds in fertilisation, the result will be a child affected with a chromosomal alteration. For this reason, when a child has been born with a chromosomal alteration, a large number of physicians will, based on the type of chromosomal alteration, recommend a chromosomal foetal study as a preventive measure in the event of a new pregnancy.
- If these errors do not occur in organs that are involved in reproduction, that is in the ovary or testis, the mosaics are called somatic cell mosaics. These mosaics are born and die in the actual individual, and consequently are not passed onto the offspring. In some cases, however, for the particular individual, they can cause cancer.
Next topic: Where are we now and where are we headed
Reviewed: 19th of January 2015